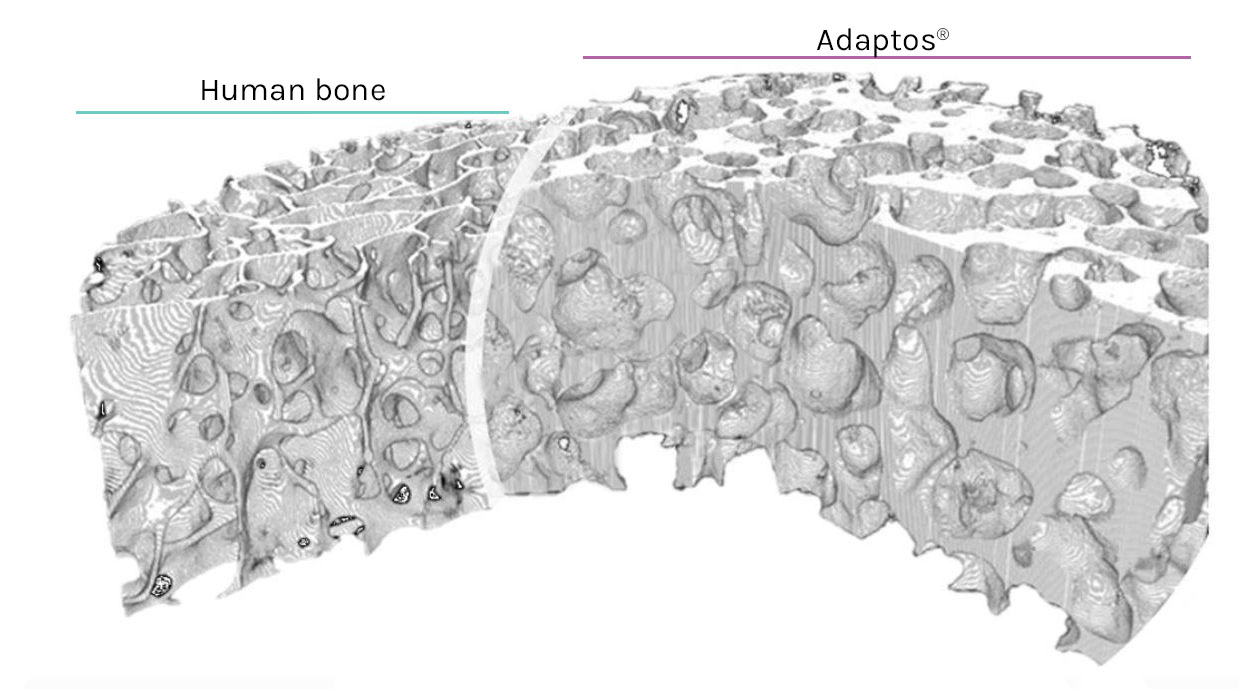
Today is the day we all have been waiting for at Biomendex. Our very first orthopaedic patient was successfully operated as a part of our randomized, controlled, multi-center clinical investigation to evaluate the safety and efficacy of Adaptos®Ortho Wedge Bone Graft Substitute in corrective knee malalignment (high tibial osteotomy).
After initial pilot study in human oral indication with encouraging preliminary results, as well as successful global market entry in veterinary practices (Adaptos®Vet has been used in nearly 1000 veterinary operations), Adaptos® is now in the real test that will help us to understand the performance capabilities of our bone graft material.
A special thanks to the HUS Helsingin yliopistollinen sairaala (HUS) research team for completing the very first operation in this clinical trial. Your dedication to the trial makes it possible for us to reach this significant milestone! In particular, we'd like to thank our HUS Principal Investigator Juuso Siren and Coordinating Investigator Mikko Manninen as well as the rest of the surgeons involved: Jan Lindahl, Joona Kalske, Kristian S., Timo Lenkkeri, Lasse Rämö, Thomas Schlenzka and Tomi Simons. And of course, we'd like to thank our Clinical Study Manager Gunilla Högnäs and CRO Labquality who have been preparing us for this trial and shall continue overseeing the progress at the sites.
Success takes grit, determination and passion – we have it!